Last updated: 18 August 2025
PN-477 Peptide Information Hub
Quick answer: PN-477 Peptide is an investigational treatment under study for weight and metabolic health. This hub explains what it is, how it may function, what the latest research shows, and how it compares with established medicines, drawing on trusted sources.
What you’ll learn
- Overview of PN-477 Peptide and its possible benefits.
- How it might influence metabolism compared with other options.
- Current research status and where trials are being run.
- Considerations around safety and evidence quality.
- Reliable places to check for updates.
What is PN-477?
PN-477 Peptide is a new therapy being investigated for weight management and related conditions. Unlike established treatments, it remains in the trial stage. Interest has grown because early information suggests it could target multiple pathways that regulate appetite and metabolism. Until research is complete, it is not available on prescription and should only be considered in a research setting.
How might PN-477 work?
Scientists are exploring how PN-477 Peptide interacts with metabolic signalling. It may affect appetite regulation, blood sugar control, and fat storage. These mechanisms are still being studied, and outcomes vary across early trial phases. Comparisons with medicines such as Semaglutide or Tirzepatide remain speculative until more data is published.
Evidence so far
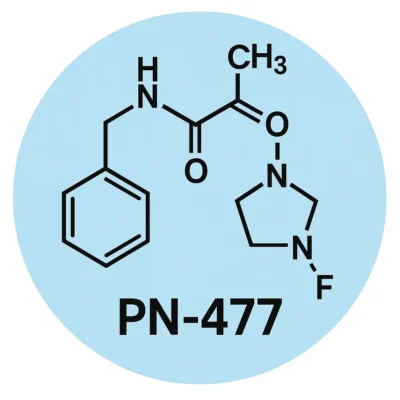
In June 2025, Protagonist Therapeutics announced the nomination of PN‑477 Peptide as a clinical development candidate. The peptide is designed as a triple agonist of GLP‑1, GIP, and glucagon receptors and is being developed in both oral and injectable formats. IND‑enabling studies are ongoing with first‑in‑human trials expected in 2026 (BioSpace press release).
Coverage in specialist outlets highlighted that PN‑477 Peptide aims to improve gastrointestinal tolerability and support a healthier fat‑to‑lean mass ratio compared with current GLP‑1 therapies (Bariatric‑News).
Ongoing research
Several PN-477 Peptide studies are listed on international trial registries. Participation in clinical research is carefully regulated, and only accredited centres can enrol patients. This ensures trial data are collected responsibly and that safety oversight is robust.
Benefits and limits
- Potential benefits: Early signs of weight loss, improved metabolic markers, broader pathway targeting.
- Limitations: Limited human data, no regulatory approval, long‑term outcomes unknown.
- Safety factors: Side effects and tolerability are still being investigated.
Future outlook
Researchers expect more results from mid‑ to late‑stage trials over the coming years. If PN-477 Peptide demonstrates strong effectiveness and safety, it could progress towards regulatory review. Even so, the approval process is lengthy, requiring evidence from large, diverse populations. This makes transparent communication of progress critical for public understanding.
Everyday context
For individuals following news on treatments like PN-477, it can help to compare it with broader lifestyle and medical options. Healthy diet, physical activity, and existing NHS‑approved treatments remain the mainstay of care until new medicines prove their value and safety. PN-477 Peptide may one day add to that toolkit, but at present it remains experimental.
Quick links
FAQs
- Is PN-477 available through the NHS?
- No. It is only accessible in clinical trials at approved centres.
- How does PN-477 compare with GLP‑1 medicines?
- It may influence additional pathways, but clear comparisons require robust head‑to‑head studies which are not yet available.
- Where can I check reliable updates?
- Regulators, academic journals, and international trial registries are the most trustworthy sources.
- Does PN-477 have known side effects?
- Potential side effects are being investigated in ongoing studies. At present, safety data are too limited to draw firm conclusions.
External references
- Protagonist Therapeutics — Press release on PN‑477 nomination
- Bariatric‑News — Coverage of PN‑477 candidate selection
- BioSpace — Industry report on PN‑477 development